 |
 |
||||||
Is Our ‘Inverted’
Retina
Really ‘Bad Design’?
Peter W. V.
Gurney
© 1999 by Answers in
Genesis. All rights reserved. Used by permission.
This
article first appeared in Vol. 13, No. 1 of the Creation Ex Nihilo
Technical Journal,
a peer-reviewed journal published by Answers in Genesis.
[Last
Modified: 14 March 2002]
he ‘inverted’ arrangement of the vertebrate retina, in which light has to pass through several inner layers of its neural apparatus before reaching the photoreceptors, has long been the butt of derision by evolutionists who claim that it is inefficient, and therefore evidence against design. This article reviews the reasons for our having the inverted retina and why the opposite arrangement (the verted retina), in which the photoreceptors are innermost and the first layer to receive incident light, would be liable to fail in creatures who have inverted retinas. I suggest that the need for protection of the retina against the injurious effects of light, particularly with the shorter wavelengths, and of the heat generated by focused light necessitates the inverted configuration of the retina in creatures possessing it.
Introduction
Evolutionists frequently maintain that the vertebrate retina exhibits a feature which indicates that it was not designed because its organisation appears to be less than ideal. They refer to the fact that for light to reach the photoreceptors it has to pass through the bulk of the retina’s neural apparatus, and presume that consequent degradation of the image formed at the level of the photoreceptors occurs. In biological terms this arrangement of the retina is said to be inverted because the visual cells are oriented so that their sensory ends are directed away from incident light (Figure 1). It is typical of vertebrates but rare among invertebrates, being seen in a few molluscs and arachnids.[1]
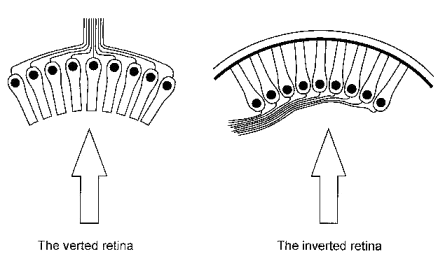
Figure 1. The two arrangements of photoreceptors. The arrows indicate the direction of incident light.
The opposite arrangement, which is more commonly seen in the invertebrates, is said to be verted, i.e. the receptive elements face the surface. This is thought to be more efficient by many evolutionists who ridicule the inverted arrangement as being ‘back-to-front’ or ‘inside-out’. However, Dawkins, a leading atheistic evolutionist, while admitting that light traversing the inverted retina is not disturbed significantly during its passage to the photoreceptors, writes as follows :
‘Any engineer would naturally assume that the photocells would point towards the light, with their wires leading backwards towards the brain. He would laugh at any suggestion that the photocells might point away, from the light, with their wires departing on the side nearest the light. Yet this is exactly what happens in all vertebrate retinas. Each photocell is, in effect, wired in backwards, with its wire sticking out on the side nearest the light. The wire has to travel over the surface of the retina to a point where it dives through a hole in the retina (the so-called ‘blind spot’) to join the optic nerve. This means that the light, instead of being granted an unrestricted passage to the photocells, has to pass through a forest of connecting wires, presumably suffering at least some attenuation and distortion (actually, probably not much but, still, it is the principle of the thing that would offend any tidy-minded engineer). I don’t know the exact explanation for this strange state of affairs. The relevant period of evolution is so long ago.’[2]
Before considering the validity or otherwise of this evolutionary viewpoint, it may be helpful to review briefly some basic ocular anatomy and terminology.
Light enters the human eye via the transparent cornea, the eye’s front window, which acts as a powerful convex lens. After passing through the pupil (the aperture in the iris diaphragm) light is further refracted by the crystalline lens. An image of the external environment is thus focused on the retina which transduces light into neural signals and is the innermost (relative to the geometric centre of the eyeball) of the three tunics of the eye’s posterior segment. The other two tunics of the eye’s posterior segment are the white tough fibrous sclera which is outermost and continuous with the cornea anteriorly, and the choroid, a pigmented and highly vascular layer which lies sandwiched between the retina and sclera.
The retina consists of ten layers (Fig. 3), of which the outermost is the dark retinal pigment epithelium (RPE) which because of its melanin pigment is opaque to light. The RPE cells have fine hair-like projections on their inner surface called microvilli which lie between and ensheath the tips of the photoreceptor outer segments. There is thus a potential plane of cleavage between the RPE and the photoreceptors which is manifested when the neurosensory retina becomes separated from the RPE, e.g. as a result of injury, a condition known as retinal detachment.
Each photoreceptor, whether rod or cone, consists of an inner and an outer segment, the former having organelles (intracellular apparatus) for manufacturing the visual pigment present in the latter. The rod and cone layer and all eight layers internal to it constitute (in distinction from the RPE) what is known as the neurosensory retina which is virtually transparent to light. By means of many complex nerve connections within the neurosensory retina, electrical impulses generated by light reaching the photoreceptors are processed and transmitted to the retina’s nerve fibre layer and thence pass up the optic nerve to the brain.
In many species for whom vision in very low levels of illumination is important, a layer of reflective crystalline material, the tapetum (Latin: carpet) is incorporated in the RPE or choroid.[3] Acting as a mirror, the tapetum reflects light which has passed between the photoreceptors, so augmenting the light bombarding the photoreceptors. Hence the proverbial ‘cat’s eyes’ when caught by a beam of light in the dark.
The Retinal Pigment Epithelium
Fundamental to understanding the inverted retina is the crucial role played by the RPE. Many of its important functions are now well known.[4],[5] Each RPE cell is in intimate contact with the tips of 20 or more photoreceptor outer segments which number over 130 million.[6] Without the RPE the photoreceptors and the rest of the neurosensory retina cannot function normally and ultimately atrophy. Thus if the neurosensory retina becomes separated (‘detached’) from the RPE the vision of the affected area of retina will deteriorate or be extinguished in time.
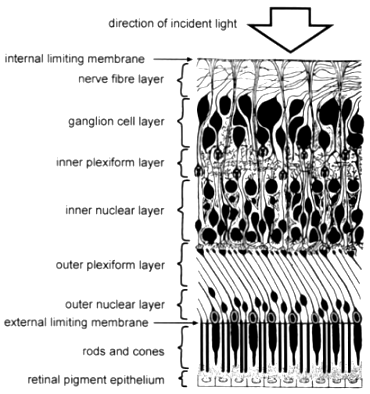
Figure 3. Diagram showing the layers of the retina (Modified from Brash J.C. [ed.], 195. Cunningham’s Textbook of Anatomy, p. 1169, by permission of Oxford University Press, Oxford).
The outer segment of a photoreceptor consists of a stack of discs containing light-sensitive photopigment. These discs are being continually formed by the inner segment from where they move in succession outwards in the outer segment towards the RPE which phagocytoses (Greek: phago, eat) them and recycles their chemical components.[7]
The RPE stores vitamin A, a precursor of the photopigments, and thus participates in their regeneration. There are four photopigments which are all bleached on exposure to light: rhodopsin (found in the rods, for night vision) and one for each of the three different types of cones (one for each of the primary colours). It synthesises glycosaminoglycans for the interphotoreceptor matrix i.e. the material lying between and separating the photoreceptors.
Besides oxygen, the RPE selectively transports nutrients from the choroid to supply the outer third of the retina and removes the waste products of photoreceptor metabolism to be cleared by the choroidal circulation. By selective pumping of metabolites and the presence of its tight intercellular junctions, the RPE acts as a barrier, called the blood-retinal barrier, preventing access of larger or harmful chemicals to retinal tissue, thereby contributing to the maintenance of a stable and optimal retinal environment.[8],[9],[10]
The RPE has complex mechanisms for dealing with toxic molecules and free radicals produced by the action of light. Specific enzymes such as the superoxide dismutases, catalases, and peroxidases are present to catalyse the breakdown of potentially harmful molecules such as superoxide and hydrogen peroxide. Antioxidants such as a-tocopherol (vitamin E) and ascorbic acid (vitamin C) are available to reduce oxidative damage.
Our photoreceptors thus continually synthesise new outer segment discs with their specific photopigments, recycling materials from used discs digested by the RPE. This prompts the question, ‘Why have such a complicated process?’ The answer must be that it is an example of biological renewal, by means of which tissues exposed to damaging chemicals, radiation, mechanical trauma, etc., are able to survive. Without self renewal, tissues such as the skin, the lining of the gut, blood cells etc would quickly accumulate fatal defects. In the same way, by the continual replacing of their discs the photoreceptors counter the relentless process of disintegration accelerated by toxic agents, particularly short wavelength light.[11],[12]
The RPE cells contain granules of the pigment melanin which absorbs scattered and excess light, so improving visual acuity (VA — i.e. the measure of sharpness of vision or of the ability to perceive as distinct two points close together). Between 25 and 33 % of all light entering the eye is absorbed by pigment granules in the RPE and the choroid.[13] The spectral absorption of melanin increases with decreasing wavelength and it is the shorter wavelengths which have the greater energy.[14] It thus also protects the photoreceptors from photic injury and is thought to function as a suppressor of photosensitized molecules, including singlet oxygen,[15] as well as a quencher of free radicals.11 Besides this, the melanin pigment of the RPE and of the choroid screens the photoreceptors from light entering the eye through the sclera.
Such intensive metabolic activity in the RPE requires a good blood supply which, as will now be considered, is provided more than adequately by the choroid lying in contact with it.
The Choroidal Heat Sink
It has been observed that the damage to photoreceptors in an experimental model is strongly related to temperature,[16] and other studies have confirmed that heat exacerbates photochemical injury. Any system designed to protect against the latter should also protect against the former.[17]
In 1980, a paper was published which explained for the first time something already known about the choroid.[18] That is, its very high rate of blood flow which far exceeds the nutritional needs of the retina, despite the latter being highly active metabolically, as indicated. This paper referred to earlier experiments showing that much less light energy was required to cause a retinal burn in dead animals than in living animals.
It went on to describe experiments with animals which demonstrated that reducing the choroidal blood flow rendered the retina more susceptible to light-induced thermal injury. The choroid takes 85% of the ocular blood flow, and the choroid is remarkable for having the highest blood flow per gram of tissue of all tissues in the body, four times greater even than that of the renal (kidney) cortex. The authors also noted that little oxygen is extracted from blood flowing through the choroid.
The choroidal capillaries (the choriocapillaris) form a rich plexus lying immediately external to the RPE, predominantly its central area, and separated from it by only a very thin membrane (Bruch’s). The absorption of excess light by the RPE produces heat in the outer retina which has to be dissipated if thermal damage to the delicate and complex biological machinery, its own and that of its neighbourhood, is to be avoided.
The authors of this study cogently argue that an important function of the choroid with its torrential blood flow (in local terms) and its close proximity to the RPE, is to act as a heat sink and cooling device. Still more fascinating are the results of further studies by the same workers indicating that there are central (via the brain), light-mediated nervous reflexes regulating choroidal blood flow, increasing the blood flow with increased illumination.[19],[20]
It is evident therefore that for the human retina to function, the presence of both the RPE and the choroid are essential. But both structures are opaque, the RPE because of its melanin and the choroid because of its blood and melanin. It follows that for light to reach the photoreceptors, both RPE and choroid have to be located external to the neurosensory retina; hence we can conclude that there are sound reasons for the inverted configuration of the human and vertebrate retina.[21],[22] Two other design features related to the inverted configuration should be considered.
The Foveola
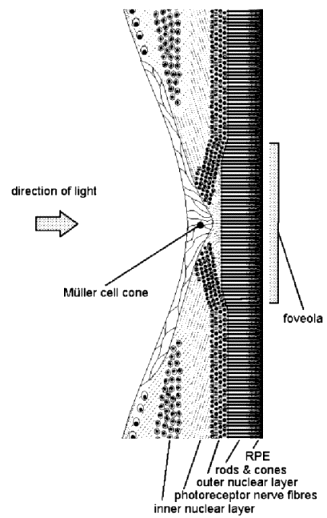
Figure 5. Diagram of the human fovea in cross section.
Although the neurosensory retina is virtually transparent apart from the blood in its very slender blood vessels, there is an additional refinement of its structure in its central region called the macula. The retina and the occipital cerebral cortex (called the visual
cortex)[23] of the brain, to which the former transmits visual information, are so organised that the VA is maximal in the visual axis (line of sight — see Fig. 2). The visual axis passes through the foveola which forms the floor of a circular pit with a sloping wall, the fovea (Latin: pit) at the centre of the macula (Figs. 2 & 5). Away from the fovea the VA diminishes progressively towards the periphery of the retina. Thus the colour photoreceptors — the cones for red, green and possibly also blue[24] — have their greatest density of 150,000 per square mm at the foveola,[25] which measures only 300–330 µm across.[26]Moreover, the foveolar cones differ from those elsewhere in being taller, more slender, perfectly straight and accurately oriented to be axial with respect to incident light, for maximal VA and sensitivity. In this area, blood vessels are absent and the retina is much thinner, being reduced to only photoreceptors (cones) with minimal supporting tissue. The inner neural elements of the neurosensory retina are displaced from the foveola radially to allow unimpeded access of light and elimination of what little scattering of light occurs elsewhere (Fig. 5). The central retina serves primarily colour and form perception while the peripheral retina is more concerned with light and motion detection and with night vision. And consistent with these functions, there is a low ratio of receptors to ganglion cells[27] (1:1.2 or more) at the fovea where optical aberrations are also minimal. By contrast, elsewhere in the retina the ratio is reversed with 4–6 cones or up to 100 rods to each ganglion cell.[28],[29]
Xanthophyll Pigment
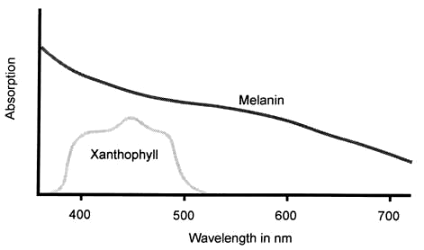
Figure 6. Ocular pigment spectral absoprtions (from various sources).
The optical system of the human eye is such that ambient light tends to fall with peak intensity on the macular area of the retina with much less on the retinal periphery. It must be significant therefore that not only is melanin more abundant in the macular region because its RPE cells are taller and more numerous per unit area than elsewhere[30] but there is also in the retina’s central area the yellow pigment xanthophyll (Greek: xanthos, yellow). In this region of the retina, xanthophyll permeates all layers of the neurosensory retina between its two limiting membranes and is concentrated in the retinal cells, both the neurons and the supporting tissue
cells.[31] Recently attention has been drawn to the presence of a collection of retinal supporting tissue cells (called Müller cells after the person who first described them) over the internal surface of the fovea and forming a cone whose apex plugs the foveolar depression (Fig. 5).[32] Besides providing structural support for the fovea, it is thought that the Müller cells, particularly in this location, act as a reservoir of xanthophyll.Retinal xanthophyll is a carotenoid, chemically related to vitamin A, whose absorption spectrum peaks at about 460 nm and ranges from 480 nm down to 390 nm (Figure. 6[33],[34],[35]).[13],[36],[37] It helps to protect the neurosensory retina by absorbing much of the potentially damaging shorter wavelength visible light, i.e. blue and violet, which is more scattered by small molecules and structures.[38] Studies have shown that the retina’s sensitivity to photic damage increases exponentially with decreasing wavelength, being six times more sensitive to ultraviolet radiation (UVR) than to blue light.[39] However, almost all of the non-ionising radiation with wavelengths shorter than 400 nm is blocked by the combination of cornea and lens,[40] leaving a remaining dangerous band of wavelengths, 420–450 nm in the blue part of the spectrum, against which xanthophyll is an effective shield.[41]
The presence of this pigment was well demonstrated in practice when ophthalmologists used to use the argon laser in which the emitted blue light was at first not subtracted; xanthophyll would absorb the blue light and produce an unwanted burn in the neurosensory retina. Modern ophthalmic argon lasers are made to emit only green light for this reason.[35]).
The Blind Spot
Because of the retina’s inverted arrangement, the axons (nerve fibres) transmitting data to the brain pass under cover of the retina’s inner surface to converge to a small area which is the optic nerve head, where they all exit the eye together as the optic nerve. The optic nerve head has no photoreceptors and so is blind, thereby producing a small blind spot in the visual field. This also has been a target of criticism by evolutionists who suggest that it can significantly disadvantage creatures that have it. As Williams puts it:
‘Our retinal blind spots rarely cause any difficulty, but rarely is not the same as never. As I momentarily cover one eye to ward off an insect, an important event might be focused on the blind spot of the other.’[42]
Notwithstanding, this issue has to be viewed in perspective: the blind spot is centred at 15° away from the visual axis (3.7 mm from the foveola) and is very small in relation the visual field of an eye, occupying less than 0.25 %.[43] As mentioned above, the further away a point in the retina is from the foveola, the less will be its VA and its sensitivity. The retina surrounding the optic nerve head, in the light-adapted state, has a VA of only about 15 % of that at the foveola.[44] We can safely infer that the theoretical risk referred to by Williams arising from the blind spot in a one-eyed person, is negligible; and, in keeping with this, it is considered safe for a one-eyed person to drive a private motor car i.e. for non-vocational purposes.
Because the two visual fields overlap to a large degree, the blind spot of one eye is covered by the other eye’s visual field. It is true that occlusion or loss of one eye is a handicap, but this is not because of the blind spot of the seeing eye for the reasons given above. Rather, and much more important to survival in a threatening situation when sight may be vital, it is the loss of stereopsis (binocular distance or depth perception) together with a moderate reduction of peripheral visual field[45] which would constitute a handicap.
However, suppose the Creator had sought to avoid the situation described by Williams by creating a very large single eye with two identical optical systems capable of converging their visual axes and two foveae far enough apart to achieve the level of stereopsis we enjoy. The result would be manifestly impractical. Indeed such a hypothetical one-eyed creature would be the more vulnerable should its only eye be injured or covered.
Human and Animal Vision
The human eye’s visual acuity, while good, is not as high as that of some animals, such as birds, whose retinae are also inverted. But variations in performance are related to the demands of the individual creature’s way of life.
The human visual system cannot register motion as accurately and sensitively as that of a fly, but if it did, we would see all fluorescent lighting and television flickering continually. We cannot see at night as well as a cat, but we surpass it in some other areas. For example, cats have no colour vision. The human eye represents an excellent balance between versatility and performance, which has enabled man’s astonishing technological achievements in antiquity. Latterly, man’s capacity to construct devices to see the far distant, the microscopic, and to see in the darkest night, has augmented the practical scope of our vision to exceed that of any other creature.
The Verted Retinae of Invertebrates
Some evolutionists claim that the verted retinae of cephalopods, such as squids and octopuses, are more efficient than the inverted retinae found in vertebrates.[46] But this presupposes that the inverted retina is inefficient in the first place. As shown above, evolutionists have failed to demonstrate that the inverted retina is a bad design, and that it functions poorly; they ignore the many good reasons for it.
Also, they have never shown that cephalopods actually see better. On the contrary, their eyes merely ‘approach some of the lower vertebrate eyes in efficiency’[47] and they are probably colour blind.[48] Moreover, the cephalopod retina, besides being ‘verted’, is actually much simpler than the ‘inverted’ retina of vertebrates; as Budelmann states, ‘The structure of the [cephalopod] retina is much simpler than in the vertebrate eye, with only two neural components, the receptor cells and efferent fibres’.[49] It is an undulating structure with ‘long cylindrical photoreceptor cells with rhabdomeres consisting of microvilli’,[50] so that the cephalopod eye has been described as a ‘compound eye with a single lens’.[51] The rhabdomeres act as light guides, and their microvilli are arranged such that the animal can detect the direction of polarized light — this foils camouflage based on reflection.
Finally, in their natural environment cephalopods are exposed to a much lower light intensity than are most vertebrates and they generally live only two or three years at the most. Nothing is known about the lifespan of the giant squid; in any case it is believed to frequent great depths at which there is little light.[52] Thus for cephalopods there is less need for protection against photic damage. Being differently designed for a different environment, the cephalopod eye can function well with a ‘verted’ retina.[53]
Summary and Conclusion
Although it would appear at first sight that the inverted arrangement of the retina has disadvantages and is inefficient, in reality these objections amount to little. Even evolutionists concede that the inverted retina serves those creatures that possess it, very well;41 it affords them superb visual acuity. We have reviewed the necessity for this arrangement which turns on the nature of the photoreceptors.
Summarizing:
Light at various wavelengths is capable of very damaging effects on biological machinery. The retina, besides being an extremely sophisticated transducer and image processor, is clearly designed to withstand the toxic and heating effects of light. The eye is well equipped to protect the retina against radiation we normally encounter in everyday life. Besides the almost complete exclusion of ultraviolet radiation by the cornea and the lens together, the retina itself is endowed with a number of additional mechanisms to protect against such damage:
The retinal pigment epithelium produces substances which combat the damaging chemical by-products of light radiation.
The retinal pigment epithelium plays an essential part sustaining the photoreceptors. This includes recycling and metabolising their products, thereby renewing them in the face of continual wear from light bombardment.
The central retina is permeated with xanthophyll pigment which filters and absorbs short-wavelength visible light.
The photoreceptors thus need to be in intimate contact with the retinal pigment epithelium, which is opaque. The retinal pigment epithelium, in turn, needs to be in intimate contact with the choroid (also opaque) both to satisfy its nutritional requirements and to prevent (by means of the heat sink effect of its massive blood flow) overheating of the retina from focused light.
If the human retina were ‘wired’ the other way around (the verted configuration), as evolutionists such as Dawkins propose,2 these two opaque layers would have to be interposed in the path of light to the photoreceptors which would leave them in darkness!
Thus I suggest that the need for protection against light-induced damage, which a verted retina in our natural environment could not provide to the same degree, is a major, if not the major reason for the existence of the inverted configuration of the retina.
Acknowledgement
I am indebted to Dr J.D. Sarfati for his suggestions, for information on physical chemistry and for his contribution concerning cephalopod and other animal eyes in the preparation of this article.
References
, where h is Planck’s Constant and c is the speed of light in a vacuum. [RETURN TO TEXT][1] Duke-Elder, S., 1958. System of Ophthalmology, Henry Kimpton, London, vol. 1, p. 147. [RETURN TO TEXT]
[2] Dawkins, R., 1986. The Blind Watchmaker: Why the evidence of evolution reveals a universe without design. W.W. Norton and Company, New York, p. 93. [RETURN TO TEXT]
[3] Duke-Elder, Ref. 1, pp. 608–609. [RETURN TO TEXT]
[4] Hogan, M. J., Alvarado, J. A., Weddell, J. E., 1971. The Retina. In Histology of the Human Eye, pp. 393–522. W.B. Saunders, Philadelphia. As cited in Tasman W., Jaeger E.A. (eds), 1998. Foundations of Clinical Ophthalmology, Lippincott–Raven, New York, vol. 1, ch. 21. [RETURN TO TEXT]
[5] Zinn, K.M., Benjamin-Henkind, J., 1979. Anatomy of the human retinal pigment epithelium. In Zinn, K. M., Marmot, M. F. (eds.), The Retinal Pigment Epithelium, pp. 3–31. Harvard University Press, Cambridge, MA. As cited in Tasman W., Jaeger E. A. (eds.), Ref. 4. [RETURN TO TEXT]
[6] The photoreceptors are of two types: the rods which number about 125 million and the cones about 6.5 million. [RETURN TO TEXT]
[7] LaVail, M.M., 1983. Outer segment disc shedding and phagocytosis in the outer retina. Trans. Ophthalmol. Soc. UK 103:397. [RETURN TO TEXT]
[8] Steinberg, R.H., 1986. Research update: report from a workshop on cell biology of retinal detachment. Exp. Eye Res. 43:696–706. [RETURN TO TEXT]
[9] Törnquist, P., Alm, A., Bill, A., 1990. Permeability of ocular vessels and transport across the blood-retinal barrier. Eye 4: 303–309. [RETURN TO TEXT]
[10] Grierson, I., Hiscott, P., Hogg, P., Robey, H., Mazure, A., Larkin, G., 1994. Development, repair and regeneration of the retinal pigment epithelium. Eye 8: 255–262. [RETURN TO TEXT]
[11] Kennon Guerry, R., Ham, W.T., Mueller, H.A. 1998. Light toxicity in the posterior segment. In Tasman W., Jaeger EA. (eds.), Clinical Ophthalmology, Lippincott-Raven, New York, vol. 3, ch. 37. [RETURN TO TEXT]
[12] Young, R.W., 1982. The Bowman Lecture: Biological renewal: Applications to the eye. Trans. Ophthalmol. Soc. UK 102:42–67. [RETURN TO TEXT]
[13] Geeraets, W.J., Williams, R.C., Chan, G., Ham, W.T., Guerry, D., Schmidt, F.H., 1960. The loss of light energy in retina and choroid. Arch. Ophthalmol. 64:158. As cited by Parver, L.M. et al., Ref. 18. [RETURN TO TEXT]
[14] Photon energy (E) is inversely proportional to wavelength (l): E = hc/l
[15] Spectroscopic terms like ‘singlet’, ‘doublet’, ‘triplet’ etc. refer to the number of possible orientations of the total electronic spin of the molecule in a magnetic field. The ground (lowest energy) state of the O2 molecule is a triplet state (3Sg–) with two unpaired electrons. But when excited by a photon, it moves into a higher energy (thus more reactive) singlet state (1Dg) with no unpaired electrons. [RETURN TO TEXT]
[16] Noell, W.K., Walker, V.S., Kang B.S. et al., 1966. Retinal damage by light in rats. Invest. Ophthalmol. 5:450. [RETURN TO TEXT]
[17] Friedman, E., Kuwubara, T., 1968. The retinal pigment epithelium: IV. The damaging effects of radiant energy. Arch. Ophthalmol. 80:265–279. [RETURN TO TEXT]
[18] Parver, L.M., Auker, C., Carpenter, D.O., 1980. Choroidal blood flow as a heat dissipating mechanism in the macula. Am. J. Ophthalmol. 89:641–646. [RETURN TO TEXT]
[19] Parver, L.M., Auker, C., Carpenter, D.O., 1983. Choroidal blood flow: III. Reflexive control in the human. Arch. Ophthalmol. 101:1604. [RETURN TO TEXT]
[20] Parver, L.M., 1991. Temperature modulating action of choroidal blood flow. Eye 5:181. [RETURN TO TEXT]
[21] Wieland, C., 1996. Seeing back to front: Are evolutionists right when they say our eyes are wired the wrong way? Creation Ex Nihilo 18(2):38–40. [RETURN TO TEXT]
[22] Anon., 1996. An eye for creation: an interview with eye disease researcher Dr G. Marshall, University of Glasgow, Scotland. Creation Ex Nihilo 18(4):19–21; online at http://www.answersingenesis.org/. [RETURN TO TEXT]
[23] The cerebral cortex is the grey cellular mantle (1–4 mm thick) forming the entire surface of the cerebral hemisphere of mammals. In man and the primates, part of the occipital cortex (at the posterior pole of each hemisphere) is specialised to receive signals from the two retinas and not until this level is reached by retinal signals is there conscious visual perception. The central 1.5 mm of the retina (the macula) has disproportionate representation in the visual cortex, amounting to about half of its area. [RETURN TO TEXT]
[24] The cones for blue are much less numerous than those for red and for green and it is now thought that they may not be present in the foveola. [RETURN TO TEXT]
[25] Osterberg, G., 1935. Topography of the layer of rods and cones in the human retina. Acta Ophthalmol. (suppl.) 6:1. As cited in Tasman W., Jaeger E.A. (eds), Ref. 4, vol. 1, ch. 21. [RETURN TO TEXT]
[26] The foveola subtends an angle of about 20 minutes of arc at the nodal point of the eye while the normal resolving power of the eye or the angle subtended by the minimum perceivable separation of two points is 1 minute of arc. [RETURN TO TEXT]
[27] Visual signals arising in the receptors are relayed in the retina first via the bipolar cells in the inner nuclear layer and then via the ganglion cells whose axons or nerve fibres form the nerve fibre layer of the retina. [RETURN TO TEXT]
[28] Curio, C.A., Allen, K.A., 1990. Topography of ganglion cells in human retina. J. Comp. Neurol. 300:5. As cited in Tasman W., Jaeger E. A. (eds), Ref. 4, vol. 1, ch. 19. [RETURN TO TEXT]
[29] Schein, S.J., 1988. Anatomy of macaque fovea and spatial densities of neurons in foveal representation. J. Comp. Neurol. 269:479. Cited in: Tasman W., Jaeger E.A. (eds), ref. 4, vol. 1, ch. 19. [RETURN TO TEXT]
[30] Streeten, B.W., 1969. Development of the human retinal pigment epithelium and the posterior segment. Arch. Ophthalmol. 81:383–394. [RETURN TO TEXT]
[31] Identifying the precise location of xanthophyll, i.e. the layers and structures in which it is present within the neurosensory retina has proved difficult for investigators but there is a consensus for what is given here. [RETURN TO TEXT]
[32] Gass, J.D.M., 1999. Müller Cell Cone, an Overlooked Part of the Anatomy of the Fovea Centralis. Arch Ophthalmol. 117:821–823. [RETURN TO TEXT]
[33] This graph is based on information from various sources as indicated by the references. Some absorption curves for melanin show an apparent fall-off at the short wavelength end of the light spectrum but this is caused by reduced transmission of short wavelength radiation by the ocular media (the cornea and more so the crystalline lens), rather than by a decrease in absorption by melanin granules. [RETURN TO TEXT]
[34] Duke-Elder, Ref. 1, pp. 118–125. [RETURN TO TEXT]
[35] Sabates, F.N., 1997. Applied laser optics: Techniques for retinal laser surgery. In Tasman W., Jaeger E. A. (eds), 1997. Clinical Ophthalmology, Lippincott-Raven, New York, vol. 1, ch. 69A. [RETURN TO TEXT]
[36] Nussbaum, J.J., Pruett, R.C., Delori, F.C., 1981. Historic perspectives. Macular yellow pigment. The first 200 years. Retina 1:296–310. [RETURN TO TEXT]
[37] Duke-Elder, S. (ed), 1961. Ref. 1, vol. 2, p. 264. [RETURN TO TEXT]
[38] The degree of scattering is inversely proportional to the fourth power of the wavelength. [RETURN TO TEXT]
[39] Ham, W.T. Jr., Mueller, H.A., Ruffolo, J.J. Jr. et al., 1982. Action spectrum for retinal injury from near-ultraviolet radiation in the aphakic monkey. Am. J. Ophthalmol. 93:299 [The term aphakia means absence of the lens in the eye. The eyes of monkeys were subjected to the experiments after their lenses had been removed.] [RETURN TO TEXT]
[40] Boettner, E. A., Wolter, J. R., 1962. Transmission of the ocular media. Invest. Ophthalmol. Vis. Sci 1:776. Cited in: Tasman W., Jaeger E. A. (eds), Ref. 11, vol. 5, ch. 55. [RETURN TO TEXT]
[41] The eyes are further shielded from excessive exposure to light and UVR by anatomical features: the normal horizontal orientation of the eyes when in the upright posture, the eyebrows (particularly for those with deep-set eyes), the nose and the cheeks. But any natural defence system can be overwhelmed and so it is sensible when necessary (just as it is to wear extra clothing in cold weather) to wear or use extra protection against UVR and blue light; all the more is this so with the depletion of our ozone layer. [RETURN TO TEXT]
[42] Williams, G.C., 1992. Natural Selection: Domains, Levels and Challenges, Oxford University Press, Oxford, pp. 72–73. [RETURN TO TEXT]
[43] Traquair, H.M., 1938. An Introduction to Clinical Perimetry, The C V Mosby Co., St Louis. [RETURN TO TEXT]
[44] Wertheim, Z., 1894. Psychol. Physiol. Sinnes. 7:172. Cited in: Duke-Elder, S. (ed.), 1968. Ref. 1, vol. 4, p. 611. [RETURN TO TEXT]
[45] The reduction of overall visual field with the loss or occlusion of one eye amounts to 20–25 % with the seeing eye looking straight ahead, mainly on account of the nose. The field of each eye is normally restricted by the facial contours mentioned in endnote 40. The field loss caused by the nose is largely recovered if the subject turns the head a little towards the blind side; this is often done unconsciously by a one-eyed person when looking intently. [RETURN TO TEXT]
[46] Diamond, J. 1985. Voyage of the Overloaded Ark. Discover, June, pp. 82–92. [RETURN TO TEXT]
[47] Mollusks. Encyclopædia Britannica 24:296-322, 15th ed., 1992; quote on p. 321. [RETURN TO TEXT]
[48] Hanlon, R.T., and Messenger, J.B., 1996. Cephalopod Behaviour, Cambridge University Press, Cambridge, New York, p. 19. [RETURN TO TEXT]
[49] Budelmann, B.U., 1994. Cephalopod sense organs, nerves and brain. In Pörtner, H.O., O’Dor, R.J. and Macmillan, D.L., ed. 1994. Physiology of cephalopod molluscs: lifestyle and performance adaptations, Gordon and Breach, Basel, Switzerland, p. 15. [RETURN TO TEXT]
[50] Sensory Reception. Encyclopædia Britannica 27:114–221, 15th ed., 1992; quote on p. 147. [RETURN TO TEXT]
[51] Budelmann, Ref. 48, p. 15. [RETURN TO TEXT]
[52] Mollusks, Ref. 46, p. 319. [RETURN TO TEXT]
[53] Wieland, Ref. 21, endnote 6. [RETURN TO TEXT]
Peter W.V. Gurney qualified in medicine at the University of Bristol, England in 1960. He spent nearly six years as a medical missionary working amongst Muslims in Pakistan, Aden, Ethiopia and Eritrea before returning to the UK to specialize in ophthalmology and completed his training at Moorfields Eye Hospital, London. He is a fellow of the Royal Colleges of Surgeons and of Ophthalmologists, and has practised as a consultant ophthalmologist in the West Midlands since 1980, retiring from full time service in 1998. [Return to text]
Home | Feedback | Links | Books | Back to Top
This site has received over

visits since November 1997
© TrueOrigin Archive. All Rights Reserved.
powered by Tim Wallace Technologies