|
|
Spinal cord compression by degenerative spine disease is one of the more common causes of myelopathy, however tumors or other masses can also cause myleopathies. Intraspinal tumors may originate in the substance of the spinal cord (intramedullary tumors) or compress it from the outside (extramedullary tumors). Extramedullary tumors may be inside the dura mater (intradural) or outside the dura mater (extradural). Some tumors are dumbbell shaped, with intra and extra spinal components. Although the case history may provide clues to the pathologic nature of the tumor, compression from a mass results in the clinical syndrome of myelopathy. Compression may often involve a radicular component (radiculomyelopathy).
The symptomatology (symptoms and signs) of spinal cord compression consists of sensory (pain, numbness and paresthesia), motor and autonomic disturbances, the nature and extend of which is related to:
* the level that is compressed - high or low cervical, high and low thoracic, lumbosacral
* the direction from which the compression originates; from without or from within the spinal cord, posterior, lateral or anterior
* the speed with which the compression is accomplished
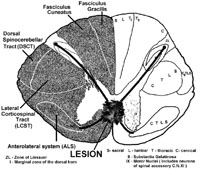
Brown-Sequard, one of the founders of clinical neurology and neurophysiology, noted in the latter half of the XIXth century, that extramedullary tumors that compress the spinal cord from its lateral or antero-lateral aspect, cause, in the early stages, impairment of pain and temperature sensation on the side CONTRALATERAL to the tumor, and weakness and spasticity on the side IPSILATERAL to the lesion.
Since that observation it has been known that damage of the anterolateral system (spinothalamic tract; ALS) in the spinal cord causes impairment or loss of pain and temperature sensation CONTRALATERAL to the lesion, and that damage of the corticospinal tract in the spinal cord (lateral corticospinal tract; LCST) results in upper motor neuron syndrome IPSILATERAL to the lesion.
Sensory abnormalities
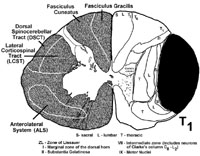
The Anterolateral System (spinothalamic tract)
Extra-medullary tumors
As you know, spinal cord pathways are somatotopically organized.
In the ALS (spinothalamic), the lowermost segments (sacral, lumbar) are
represented by fibers that lie closest to the outer surface of the spinal
cord (laterally). These fibers are most vulnerable to anterolateral
extramedullary compression. The loss of pain and temperature appears
here first, and is most complete, in the sacral dermatomes. As the
tumor expands, the sensory loss gradually ascends towards the site of the
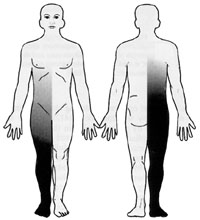
lesion. For example, anterolateral extramedullary compression at
T1 first affects fibers in the most lateral part of the ALS, i.e. the fibers
that arose from the dorsal horn cells in the contralateral sacral spinal
cord. The lesion at spinal level T1 then moves medially in the ALS
to involve axons that arose from dorsal horn neurons in the contralateral
lumbar, and thoracic dorsal horn. Thus the pain and temperature deficit
is designated as ASCENDING TOWARD THE LESION (which is at spinal
level T1; sacral first, thoracic last). Of course, the level of pain
and temperature impairment DOES NOT reach the level of the lesion (T1).
As discussed earlier in this series, the lesion (tumor) is found several
segments ABOVE the highest level of the pain and temperature deficit.
Thus, in a lesion of the ALS or spinothalamic tract at T1, the pain and
temperature loss reaches only as high as T3. No FIBERS in the ALS
at spinal level T1 contain information conveyed into the spinal cord from
spinal nerves T1 or T2. Such information will be conveyed in the
ALS above the lesion (at spinal levels C7 and C8 respectively). We
know this from observations on patients following complete cord transactions
and lateral cordotomy for pain management.
 |
 |
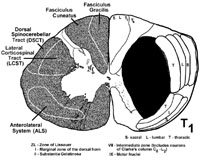
Intra-medullary tumors
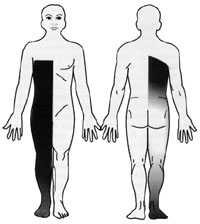
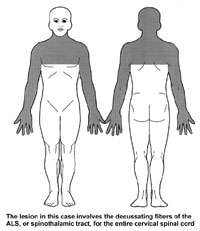
Tumors that start within one half of the spinal cord tend to compress the inner parts of the ALS or spinothalamic tract that contain the fibers that had just crossed over, (i.e. from cells in the opposite dorsal horn at that spinal segment). Therefore in patients with intramedullary tumors, the pain and temperature loss starts near the level of the tumor (approximately two levels below). As the tumor grows laterally within the ALS, the pain and temperature deficit involves LOWER regions of the body, that is, the pain and temperature loss DESCENDS in relationship to the location of the lesion. This results in the sparing of the sacral area (sacral sparing) before the sensory loss is complete (see below).
 |
 |
 |
 |
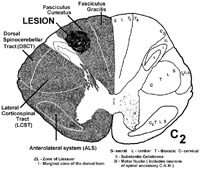
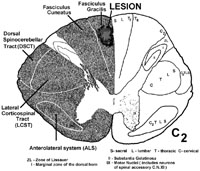
Dorsally situated tumors compress the dorsal or posterior column first, and cause paresthesia (funny sensations), numbness and impairment of 2 point discrimination, vibration and conscious proprioception on the side of the tumor. Remember, the dorsal columns are somatotopically organized, such that lateral spinal cord compression results in unilateral damage to fibers arising from dorsal root ganglia close to the lesion, whereas central or medial dorsal spinal cord compression tends to damage fibers from dorsal root ganglia relatively far from the lesion site. For instance, a lesion laterally within the dorsal columns at C2 will damage dorsal column fibers from C2 (close to the lesion site at C2) and below. In contrast, a lesion medially within the dorsal columns at C2 will damage sacral fibers (far from the lesion site at C2).
Damage to the dorsal columns results in loss of 2pt. discrimination, vibration, and proprioception. Dorsal column lesions may cause a symptom called the "Lhermitte sign." The patients experience an electric shock like sensation running through the back and limbs upon flexion of the neck. This is usually associated with lesions in the cervical cord (compression, multiple sclerosis, subacute combined degeneration from B12 deficiency). Flexing the neck stretches the dorsal part of the cord, thus irritating some of the sick or dying fibers in the fasciculi gracilis and cuneatus conveying pathological signals.
 |
 |
The lateral corticospinal tract (LCST)
Damage of the LCST in the spinal cord results in effects ipsilateral to the lesion. Like other tracts, the corticospinal tract has a somatotopic arrangement, with the most lateral fibers subserving the lower limbs.
Slow compression of the corticospinal tract causes an impairment
of motor function called upper motor neuron syndrome or lesion (UMNL).
The signs that characterize this syndrome are muscle weakness, increase
in muscle tone and abnormal reflexes, although the chief complaint may
be clumsiness of the hand. The physician evaluates muscle tone by
(for example) extending a flexed arm or flexing an extended leg.
An increase of muscle tone during these passive movements is referred to
as "spasticity." This tone increase is rate dependent (more resistance
the faster you try to stretch the muscle) and predominates in the flexors
of the upper limbs and the extensors of the lower limbs. Muscle weakness
is most marked in the abductors of the fingers and the extensors of the
upper limbs and the flexors of the lower limbs. Plantar flexion is
an extending (antigravity) movement (stand on your toes!!), and the power
of plantar flexion is preserved, whereas dorsiflexion of the foot is impaired.
In patients with chronic lateral corticospinal tract damage, spasticity
is the dominant feature, whereas weakness may be mild.
|
|
The physician evaluates muscle stretch reflexes by tapping on tendons. Following UMN lesions such reflexes are usually increased. These "deep tendon" reflexes, i.e. tap on a tendon to initiate the reflex, are really muscle stretch reflexes. The stretch activates IA afferents that respond to stretch of the nuclear bag fiber in the muscle spindle. Brisk or hyperactive muscle stretch reflexes elicited by tendon tapping may occur in normal individuals who do not have spasticity, (remember spasticity is increased tone found during passive movement of a limb, not when tapping a tendon).
The result of an upper motor neuron lesion affecting the legs is difficulty in walking. UMNL of the upper extremity results in a stiff, clumsy and weak hand.
Immediately following a lesion of the LCST (trauma, ischemia) there is "spinal shock." All voluntary (LCST) and reflex (muscle stretch) movements are lost. There is also loss of all sensory modalities and urinary retention (to be discussed later). The sensory deficits do not change, but with time the flaccid weakness is converted into a spastic upper motor neuron syndrome. The first response to stimulation in the course of this transformation occurs in the large toe in response to stimulation of the sole of the foot. Normally, the big toe plantar flexes (goes down). This is called the plantar (flexor) response. In UMN lesions the plantar response is abnormal because the big toe goes up (extension). This is called a Babinski sign and we say the plantar response is extensor, versus a normal response about which we say the plantar response is flexor.
The Babinski sign is one of the cutaneous reflexes (as opposed to the muscle stretch reflex) and it is pathognomonic (pathognomonikos = skilled in diagnosing) of an UMN lesion.
Ventral (anterior) horn cells.
Loss of ventral or anterior horn cells or the ventral roots result
in a lower motor neuron (LMN) lesion. You are already familiar with
the features of lower motor neuron lesions. Compression of the cervical
or lumbosacral spinal cord may be accompanied by significant lower motor
neuron deficits. This is usually due to direct compression of the ventral
horn cell area or the ventral root.
Autonomic disturbances
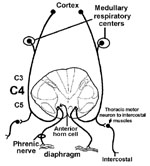
Respiration
The tracts subserving voluntary (from the cortex) and automatic (from medullary respiration centers; to be discussed in your Physiology course) breathing, travel in the ventral funiculus but are difficult to identify. If this information on both (bilateral) sides of the spinal cord does not reach the ventral horn neurons innervating the diaphragm (C3, C4, C5 give rise to the phrenic nerve) and intercostal muscles (thoracic cord ventral horn cells) the patient will die if not put on a respirator.
Severe respiratory compromise involving bilateral lesions usually results from high cervical cord injuries or anterior horn cell loss from degenerative diseases like Amyotrophic Lateral Sclerosis. For example, a bilateral lesion of the ventral funiculus at C1 could interrupt all descending inputs to cells at C3-C5 that innervate the diaphragm via the phrenic nerves. Such a lesion also interrupts the descending input to the cells in the cervical and thoracic cord that innervate the neck and intercostal muscles which assist in respiration.

Normal bladder function depends on the coordinated activity of the bladder detrussor (smooth muscle) and the sphincter muscles (internal and external sphincters and muscles of pelvic floor). The actual act of voiding is under the control of higher cortical centers that develops as continence (continere = to hold together) and is achieved in early childhood. Incontinence occurs when neuroanatomic pathways that innervate the bladder are interrupted or when there are physical problems with the pelvic floor and sphincter muscles. Incontinence is an important symptom, and if it occurs in association with other neurological deficits that localize to the spinal cord, needs to be investigated aggressively.
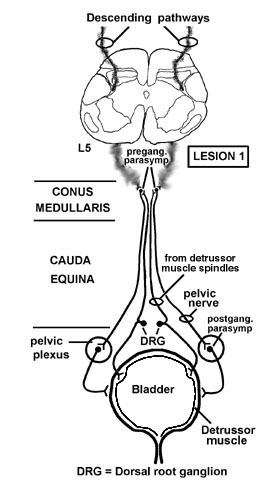
The important points are that the bladder is controlled by areas of the brain that send axons down the spinal cord, traveling just medial to the LCST. These bilateral projections terminate on preganglionic parasympathetic neurons at S2, S3 and S4. The preganglionic parasympathetic neurons send their axons out the ventral roots of S2, S3 and S4 to synapse on postganglionic parasympathetic cells in ganglia near the bladder. These postganglionic parasympathetic cells in turn innervate the detrussor (smooth) muscle of the bladder for voiding.
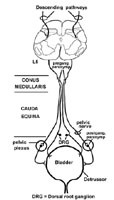
Think about what happens right after a lesion of the LCST. Spinal shock and flaccidity, right? This is similar to what happens when the descending pathways involved in bladder control are cut, only it has to be a bilateral lesion (as with respiration). For example, following a bilateral lesion of the entire spinal cord at C2 the detrussor initially becomes flaccid (like arm and leg muscles following a lesion of the LCST) and this results in urinary retention. There may be overflow incontinence when the bladder cannot physically hold any more urine. With time (or if the lesion is slow to affect the cord), spasticity develops and the bladder contracts with small degrees of stretch (analogous to an increased muscle stretch reflex in the arm and leg following a lesion of the LCST). This causes urinary frequency and urgency; whenever the bladder fills a little, the increased stretch carried by afferents activate the parasympathetic motor neurons that control the detrussor and thus intermittent voiding. The bladder is spastic. Therefore, in acute lesions of the spinal cord rostral to the sacral cord (UMNL), two things occur. First there is a flaccid bladder (acute), then later there is a spastic bladder (chronic).
Lesion 2 - Lower Motor Neuron Lesion: (This is easier.)
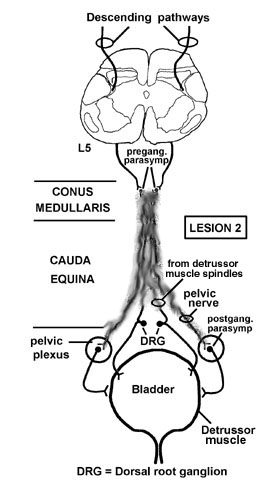
Remember, lesions of the spinal cord rostral to the sacral cord result first in a flaccid (atonic; acute) bladder, followed by a spastic (chronic) bladder. Lesions from S1 down, and involving all of the various nerves, result in ONLY a flaccid bladder.